Why Understanding Isopropanol's Boiling Point Matters
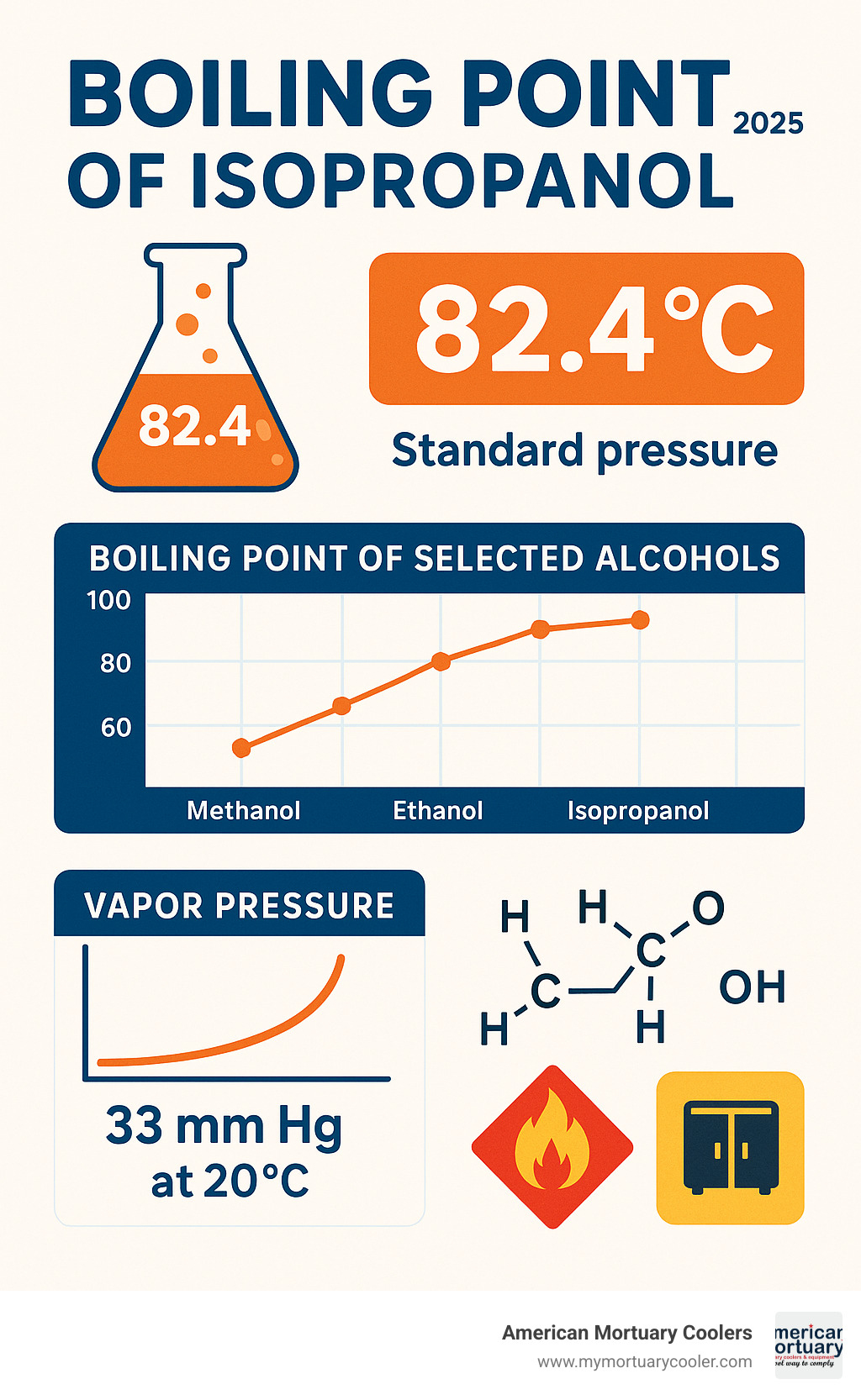
The boiling point of isopropanol is 82.4°C (180.3°F) at standard atmospheric pressure. This key physical property determines how this common alcohol behaves in everything from laboratory distillations to industrial cleaning processes.
Quick Facts:
- Temperature: 82.4°C (180.3°F) at 1 atmosphere
- Vapor pressure: 33 mm Hg at 20°C
- Molecular weight: 60.1 g/mol
- Compared to ethanol: 4°C higher (ethanol boils at 78.4°C)
Whether you're working in a funeral home laboratory, managing chemical storage, or selecting solvents for preservation work, knowing when isopropanol transitions from liquid to vapor affects safety protocols, equipment design, and process efficiency.
The boiling point tells you critical information about volatility - how quickly the alcohol evaporates at room temperature. With a vapor pressure of 33 mm Hg at 20°C, isopropanol evaporates readily, which impacts both its antimicrobial effectiveness and fire safety requirements.
Understanding these properties becomes especially important in controlled environments like mortuaries, where proper ventilation and temperature control are essential for both safety and preservation processes.
I'm Mortuary Cooler, and through years of working with funeral home directors on custom cooling solutions, I've seen how understanding chemical properties like the boiling point of isopropanol helps optimize both safety protocols and equipment performance. This knowledge directly impacts how we design ventilation systems and storage solutions for facilities handling various preservation chemicals.
Simple boiling point of isopropanol glossary:
The Boiling Point of Isopropanol Explained
When you heat isopropanol to 82.4°C (180.3°F), something fascinating happens - the liquid suddenly starts bubbling and transforms into vapor. This temperature, known as the boiling point of isopropanol, marks the exact moment when the liquid can't stay liquid anymore at normal atmospheric pressure.
Think of it like this: at room temperature, isopropanol molecules are constantly jiggling around, but they're held together by invisible molecular forces. As you add heat, they jiggle faster and faster. When you hit 82.4°C, they're moving so energetically that these holding forces can't keep them in liquid form anymore.
The NIST Chemistry WebBook provides this precise measurement, and it's backed up by ASTM D770 specifications for reagent-grade isopropanol. This isn't just academic trivia - this exact temperature matters for anyone distilling, purifying, or working with isopropanol in professional settings.
Here's what makes this interesting: even at room temperature (20°C), isopropanol has a vapor pressure of 33 mm Hg. That means molecules are constantly escaping from the liquid surface - which is why you can smell it when you open a bottle. The alcohol is literally evaporating into the air around you.
The molecular weight of 60.1 g/mol helps explain why isopropanol behaves differently from water. Its branched three-carbon structure with that hydroxyl (-OH) group creates just the right balance of molecular attractions to boil at this specific temperature.
Standard Boiling Point of Isopropanol vs Other Alcohols
Comparing the boiling point of isopropanol to its alcohol cousins reveals some interesting patterns. Methanol boils at just 64.7°C - that's nearly 18 degrees lower than isopropanol. Ethanol comes in at 78.4°C, while tert-butanol practically ties with isopropanol at 82.6°C.
Why these differences? It all comes down to molecular structure. Methanol, being the smallest with just one carbon atom, needs the least energy to vaporize. Ethanol's straight two-carbon chain requires more heat, while isopropanol's branched three-carbon structure needs even more energy to break free.
That 4°C difference between ethanol and isopropanol might seem tiny, but it's huge in the distillation world. This temperature gap allows chemists to separate these two alcohols cleanly using fractional distillation - a process that's essential in industrial isopropanol production.
The near-identical boiling points of isopropanol and tert-butanol show how molecular branching affects these properties. Both have branched structures that create similar intermolecular forces, resulting in almost the same boiling temperatures.
Measuring the Boiling Point of Isopropanol
Getting an accurate boiling point of isopropanol measurement isn't as simple as sticking a thermometer in boiling liquid. Professional labs use a reflux setup with the thermometer positioned precisely at the vapor line - not touching the liquid itself.
The secret is using high-purity isopropanol (99%+ grade) because even small impurities can throw off your results. You'll also need a calibrated thermometer accurate to within 0.1°C and careful attention to barometric pressure.
Why does pressure matter? The standard 82.4°C assumes exactly 760 mm Hg atmospheric pressure. If you're working at higher altitude where air pressure is lower, isopropanol will boil at a correspondingly lower temperature. That's why labs apply barometric correction to their measurements.
Sample purity can make or break your results. Even 1-2% water content shifts the boiling point because water and isopropanol form what's called an azeotrope. Professional labs often run a Karl Fischer water test before measuring boiling points to avoid this issue.
The NIST reference method specifies maintaining steady-state conditions - the temperature should hold constant for 2-3 minutes before you record it. This ensures you're measuring the true boiling point, not just a temperature spike.
What Changes the Boiling Point?
The boiling point of isopropanol isn't always a fixed 82.4°C - several real-world factors can shift this temperature up or down. Understanding these changes helps explain why your lab results might vary or why industrial processes need different approaches.
Pressure makes the biggest difference. Think of it this way: at lower pressure, molecules need less energy to escape the liquid surface. The Clausius-Clapeyron equation tells us that for every 10 mm Hg drop in pressure, the boiling point drops by roughly 0.5°C. This relationship is what makes vacuum distillation work - you can purify heat-sensitive compounds at much lower temperatures.
Water content creates some interesting chemistry. Pure isopropanol behaves predictably, but add water and things get complex. The two form what chemists call an azeotrope - a mixture that acts like a single compound. At 91% isopropanol and 9% water, this mixture actually boils at 80.4°C, which is lower than pure isopropanol.
This explains why your bottle of 70% rubbing alcohol evaporates differently than laboratory-grade 99% isopropanol. The water changes everything about how the mixture behaves.
Other impurities generally push the boiling point higher. Dissolved salts, oils, or any non-volatile substances make it harder for isopropanol molecules to escape into vapor form. This is basic chemistry - impurities interfere with the vaporization process.
Atmospheric Pressure Variations
Working at different elevations means dealing with different atmospheric pressures, and that directly affects the boiling point of isopropanol. At our Tennessee facility, we're close to sea level where standard pressure applies. But if you're working in Denver at 5,000 feet, the story changes completely.
High altitude drops the boiling point significantly. In Denver, atmospheric pressure sits around 630 mm Hg instead of the standard 760 mm Hg. This means isopropanol boils at about 75°C instead of 82.4°C - a full 7°C difference that affects distillation timing and evaporation rates.
Industrial facilities use this principle on purpose. Vacuum distillation systems can drop pressure to 100 mm Hg or lower, allowing isopropanol to distill at room temperature. This saves energy and prevents heat damage to sensitive compounds.
Even daily weather creates small variations. A strong storm system might drop local pressure by 20-30 mm Hg, lowering the effective boiling point by 1-2°C. For precision laboratory work, this matters more than you might think.
Purity, Water Content, and Azeotropes
Here's where the boiling point of isopropanol gets really interesting. Water doesn't just dilute isopropanol - it fundamentally changes how the mixture behaves. Instead of a simple blend, you get complex interactions that can actually lower the boiling point.
The 91% azeotrope is the key player. This specific mixture of 91% isopropanol and 9% water boils at 80.4°C, lower than either pure component alone. You literally cannot separate this mixture further using simple distillation - the vapor that comes off has the same composition as the liquid.
This creates real challenges for anyone trying to make high-purity isopropanol. Industrial processes need special tricks like extractive distillation or molecular sieves to remove that last bit of water. Simple heating won't do it.
Different grades behave completely differently. Anhydrous 99.9% isopropanol boils right at 82.4°C and works great for electronics cleaning. Reagent-grade 99% contains less than 1% water and still behaves predictably. But once you hit that 91% azeotropic composition, the boiling point drops to 80.4°C. Regular 70% rubbing alcohol boils somewhere around 78-79°C, optimized for disinfection rather than purity.
Water testing becomes crucial for quality control. Karl Fischer titration can detect water down to 0.1%, and even these trace amounts shift boiling points measurably. In pharmaceutical work or electronics manufacturing, this precision matters for both safety and product quality.
Why the Boiling Point Matters: Industry, Labs & Everyday Use
The boiling point of isopropanol directly impacts energy costs, safety protocols, and process design across numerous industries. In our experience designing custom mortuary equipment, understanding these thermal properties helps optimize ventilation systems and storage solutions.
Distillation energy requirements scale with the temperature difference between ambient conditions and the boiling point. At 82.4°C, isopropanol requires moderate heating - high enough to prevent excessive energy costs but low enough to avoid thermal stress on equipment. This makes it ideal for solvent recovery systems where economics matter.
The moderate boiling point enables effective cleaning of electronics and precision instruments. The alcohol reaches sufficient temperature for thorough cleaning while evaporating completely without leaving residues. This property makes it invaluable in funeral home laboratories where instrument cleanliness is critical.
Industrial acetone production relies on isopropanol's boiling point for the dehydrogenation process. Vaporized isopropanol at controlled temperatures reacts over catalysts to produce acetone, with the precise temperature control enabled by the predictable 82.4°C boiling point.
For facilities handling large volumes, like our clients across the Southeast and Pacific regions, understanding evaporation rates helps design proper ventilation. The relatively low boiling point means significant vapor generation even at room temperature, requiring adequate air exchange to maintain safe working conditions.
Distillation & Solvent Selection
The boiling point of isopropanol makes it an excellent choice for fractional distillation processes. The 82.4°C temperature provides clean separation from many common solvents while remaining accessible with standard heating equipment.
In solvent recovery operations, isopropanol's boiling point offers economic advantages. Unlike higher-boiling solvents that require expensive high-temperature equipment, standard steam heating or electric elements easily reach the required temperature. This reduces both capital and operating costs for recovery systems.
Fractional distillation columns designed for isopropanol typically operate with:
- Reboiler temperatures: 85-90°C
- Condenser temperatures: 5-10°C
- Reflux ratios: 2:1 to 5:1 depending on purity requirements
The separation from water requires special consideration due to azeotrope formation. Many industrial processes use a two-column system: the first removes most water, while the second uses extractive distillation with agents like cyclohexane to break the azeotrope.
Purity grades affect distillation design significantly. Moving from 95% to 99.9% purity requires exponentially more energy and theoretical plates due to the azeotropic behavior near the high-purity end.
Embalming, Cooling, and Preservation Applications
In mortuary science, the boiling point of isopropanol influences both its antimicrobial effectiveness and handling requirements. The moderate volatility provides rapid surface disinfection while evaporating quickly to prevent residue accumulation on instruments and surfaces.
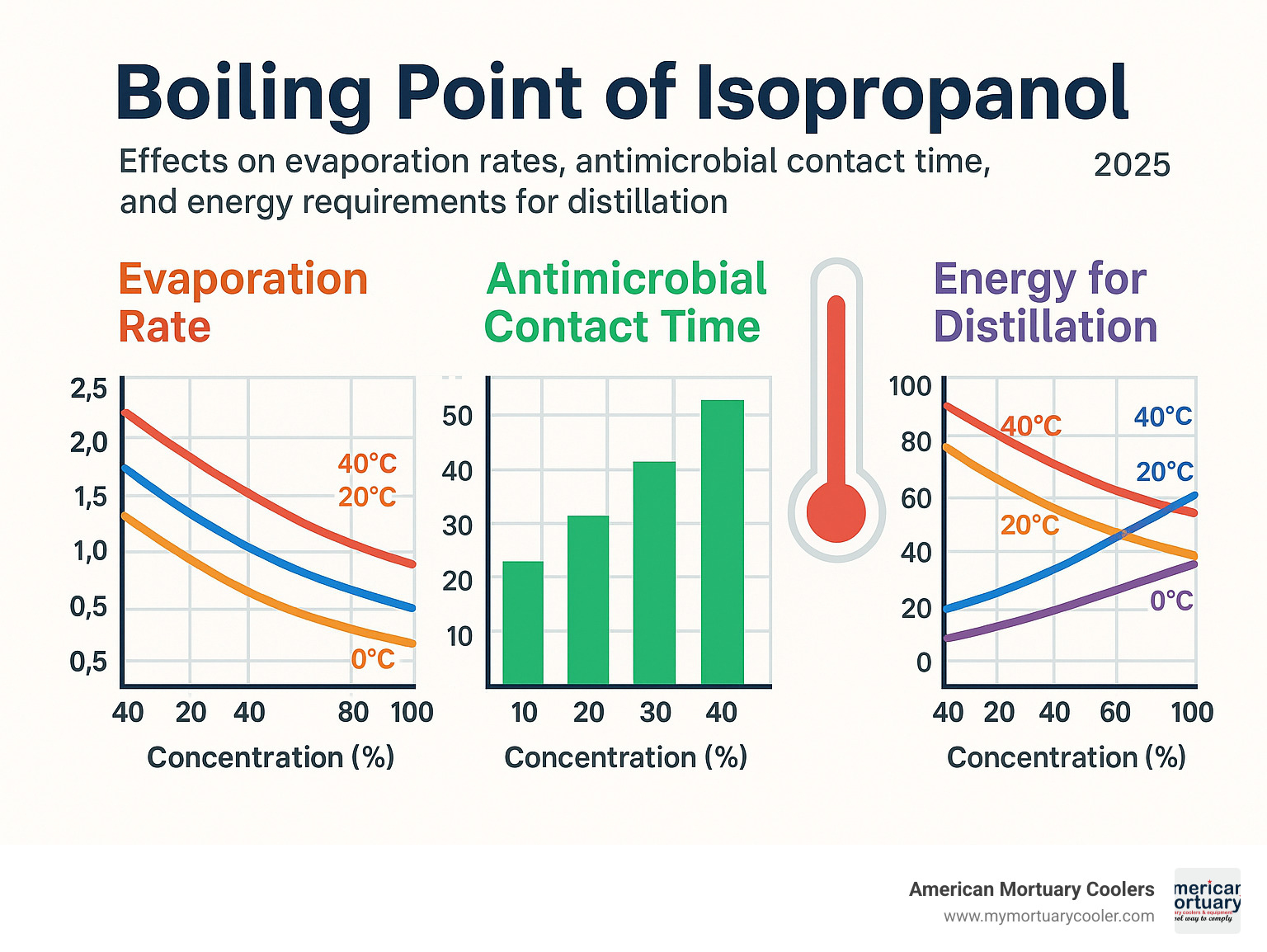
The evaporation rate, directly related to the 82.4°C boiling point, affects antimicrobial contact time. Isopropanol must remain in contact with microorganisms long enough for sterilization, but its volatility means application techniques must account for rapid evaporation, especially on warm surfaces.
Our experience designing mortuary cooling systems shows how isopropanol's thermal properties affect ventilation requirements. Facilities using significant quantities need air exchange rates calculated based on the vapor pressure at operating temperatures. The 33 mm Hg vapor pressure at 20°C means substantial vapor generation in warm environments.
Temperature control becomes crucial in preservation applications. Storage areas maintained at lower temperatures (15-18°C) significantly reduce evaporation rates, extending product effectiveness and reducing vapor exposure for workers. This knowledge influences our design of climate-controlled storage solutions.
The Science of Embalming: Chemistry, Microbiology, and Anatomy in Modern Preservation provides additional context on how chemical properties like boiling point affect preservation processes.
Understanding refrigeration interactions helps optimize storage. Our cooling systems maintain temperatures that minimize isopropanol evaporation while preserving its antimicrobial properties. More info about R-404A properties explains how refrigerant properties complement chemical storage requirements.
Safe Storage, Handling & FAQs
Understanding the boiling point of isopropanol becomes crucial when you're dealing with fire safety. Here's something that catches many people off guard: isopropanol can ignite at just 12°C (54°F) - that's its flash point, which sits way below the actual boiling point of 82.4°C. This means those vapors floating around at room temperature are already dangerous.
The workplace safety folks at OSHA know this well. They've set exposure limits at 400 ppm for an 8-hour workday, with short-term spikes allowed up to 800 ppm for 15 minutes. But here's where it gets interesting - recent studies suggest even stricter limits might be needed. The National Research Council now recommends just 1 ppm for continuous exposure in enclosed spaces like submarines.
When you're shipping or storing isopropanol, it gets the UN1219 classification as a flammable liquid. That's not just paperwork - it means specific rules about how you handle, store, and transport it. The vapor-to-air mixtures can explode across a surprisingly wide range of concentrations.
The good news? Reagent-grade isopropanol keeps for about 5 years when you store it right. The key is keeping those containers sealed tight to prevent both evaporation and water absorption from the air. Store it in cool, dry spots away from any heat sources, and make sure your electrical equipment is explosion-proof in storage areas. Don't forget about proper bonding and grounding - static electricity can provide just enough spark to cause real problems.
Volatility & Evaporation Rate
The relationship between the boiling point of isopropanol and how fast it evaporates affects your wallet and your safety. At a comfortable room temperature of 20°C, isopropanol has a vapor pressure of 33 mm Hg. To put that in perspective, about 4.3% of the air pressure in a closed container would be isopropanol vapor if it reached saturation.
This high volatility creates some real-world headaches. Containers lose their contents fast if you don't seal them properly. Work areas need continuous ventilation to keep vapors from building up to dangerous levels. Your safety gear needs to protect against vapor exposure, not just liquid splashes. And if you're designing any kind of process, you'll want to minimize open surfaces to control those evaporation losses.
Temperature makes a huge difference here. Bump the storage temperature up by 10°C, and you roughly double the evaporation rate. That's why climate control isn't just about comfort - it's about economics and safety. Through our work designing mortuary cooling systems, we've seen how proper temperature management helps clients minimize both safety risks and product losses.
When we design ventilation systems for facilities across different climates - from the humid Southeast to the dry Southwest - we use that boiling point and vapor pressure data to calculate exactly how much air exchange each location needs.
Regulatory Limits & Fire Safety
The boiling point of isopropanol at 82.4°C explains why regulatory agencies take such a serious approach to exposure and storage limits. That moderate boiling point means significant vapor generation even at normal working temperatures, creating both health concerns and fire risks.
OSHA's standards reflect this reality. They require explosion-proof electrical equipment in areas where vapors might accumulate. Proper ventilation to keep concentrations below 400 ppm becomes mandatory, not optional. You need emergency response plans that account for vapor cloud formation, and regular air monitoring in enclosed work spaces.
The fire suppression side gets tricky too. Standard firefighting foams don't work well on polar solvents like isopropanol - you need alcohol-resistant foam specifically designed for these fires. The moderate boiling point means water cooling can help control fires, but complete extinguishment requires the right type of foam.
Bonding and grounding requirements exist because of the fire hazard from vapor generation. Static electricity can provide enough energy to ignite isopropanol vapors, making proper electrical continuity essential during any transfer operations.
Frequently Asked Questions about the Boiling Point of Isopropanol
What happens to the boiling point at high altitude?
The boiling point of isopropanol drops predictably as you go higher. For every 1,000 feet of elevation, expect the boiling point to decrease by about 1.8°C due to reduced atmospheric pressure. If you're working in Denver at 5,280 feet, isopropanol boils at roughly 73°C instead of the sea-level 82.4°C. This affects distillation processes and evaporation rates throughout our Rocky Mountain service area.
Does adding salt change the boiling point?
Adding salt to isopropanol does raise the boiling point through what chemists call colligative effects - similar to salting water. However, salt doesn't dissolve as well in isopropanol compared to water, so the effect is less dramatic. A saturated salt solution might bump the boiling point up by 2-3°C. Other dissolved substances like sugars or ionic compounds have similar effects based on their concentration.
Can I safely boil isopropanol at home?
Absolutely not recommended. Boiling isopropanol at home creates serious fire and explosion risks. 12°C flash point? Vapors can ignite well before you reach the 82.4°C boiling point. Plus, concentrated vapors cause respiratory irritation and can affect your central nervous system. Any distillation work should only happen in properly equipped laboratories with appropriate safety equipment, ventilation, and fire suppression systems.
Why does rubbing alcohol seem to boil at a different temperature?
Commercial rubbing alcohol typically contains 70% isopropanol and 30% water, creating what's called an azeotropic mixture. This combination actually boils at approximately 78-79°C - lower than pure isopropanol's 82.4°C. This happens because the isopropanol-water mixture forms hydrogen bonds that reduce the energy needed for vaporization at certain concentrations.
How does the boiling point affect antimicrobial effectiveness?
The boiling point of isopropanol indirectly affects how well it kills germs through evaporation rate. Rapid evaporation due to that moderate boiling point can reduce contact time with microorganisms, potentially decreasing effectiveness. This explains why 70% isopropanol often works better than 99% - the water content slows evaporation, providing longer contact time for microbial kill while still ensuring complete evaporation.
Conclusion
Understanding the boiling point of isopropanol at 82.4°C goes far beyond memorizing a number from a chemistry textbook. This physical property shapes how we handle, store, and use isopropanol safely in everything from funeral home laboratories to industrial facilities.
The moderate boiling point creates a sweet spot for practical applications. It's low enough that standard heating equipment can easily reach distillation temperatures, keeping energy costs reasonable. Yet it's high enough to provide stability during storage and handling - you won't accidentally boil off your solvent on a hot summer day.
But here's where it gets tricky: that same 82.4°C boiling point means isopropanol is constantly trying to evaporate, even at room temperature. With a flash point of just 12°C, those vapors become a fire hazard long before the liquid starts bubbling. This is why proper ventilation isn't optional - it's absolutely critical for safe operations.
The pressure and purity effects we've discussed matter in real-world applications. If you're working at higher elevations, your isopropanol will boil at lower temperatures, affecting distillation processes and evaporation rates. Water content throws another curveball with azeotropic behavior that can surprise even experienced chemists.
For mortuary professionals, these properties directly impact daily operations. The antimicrobial effectiveness depends partly on evaporation rate - too fast, and the alcohol doesn't have enough contact time with microorganisms. Too slow, and you're left with residues on instruments and surfaces.
At American Mortuary Coolers, we've learned that temperature control makes all the difference. Our custom cooling systems help facilities across Tennessee and beyond maintain optimal storage conditions that minimize evaporation losses while preserving chemical effectiveness. Whether you're dealing with humid summers in the Southeast or dry heat in our Southwest service areas, proper climate control protects both your investment in chemicals and your staff's safety.
The science behind isopropanol's boiling point of isopropanol continues evolving as new applications emerge and safety standards tighten. Recent changes in exposure limits reflect better understanding of how volatility affects worker health over time.
Smart facility design accounts for these thermal properties from the ground up. Proper storage temperatures, adequate ventilation, appropriate fire suppression systems, and strategic equipment placement all stem from respecting what that 82.4°C boiling point tells us about how isopropanol behaves.
Looking ahead, facilities that understand these fundamentals will operate more safely and efficiently. The boiling point of isopropanol might seem like basic chemistry, but it's the foundation for making smart decisions about equipment, procedures, and safety protocols.
For funeral homes and laboratories needing specialized solutions for chemical storage and handling, our team designs equipment with these thermal properties in mind. We know that good engineering starts with understanding the science. More info about mortuary cooler solutions and how proper temperature control creates safer, more efficient preservation environments.

















